Glory Tips About How To Find Out The Valence Electrons
![How To Determine The Number Of Valence Electrons In An Element, Ion, Or Molecule [Quick And Easy] - Youtube](https://i.ytimg.com/vi/WN0uDSdrWoY/maxresdefault.jpg)
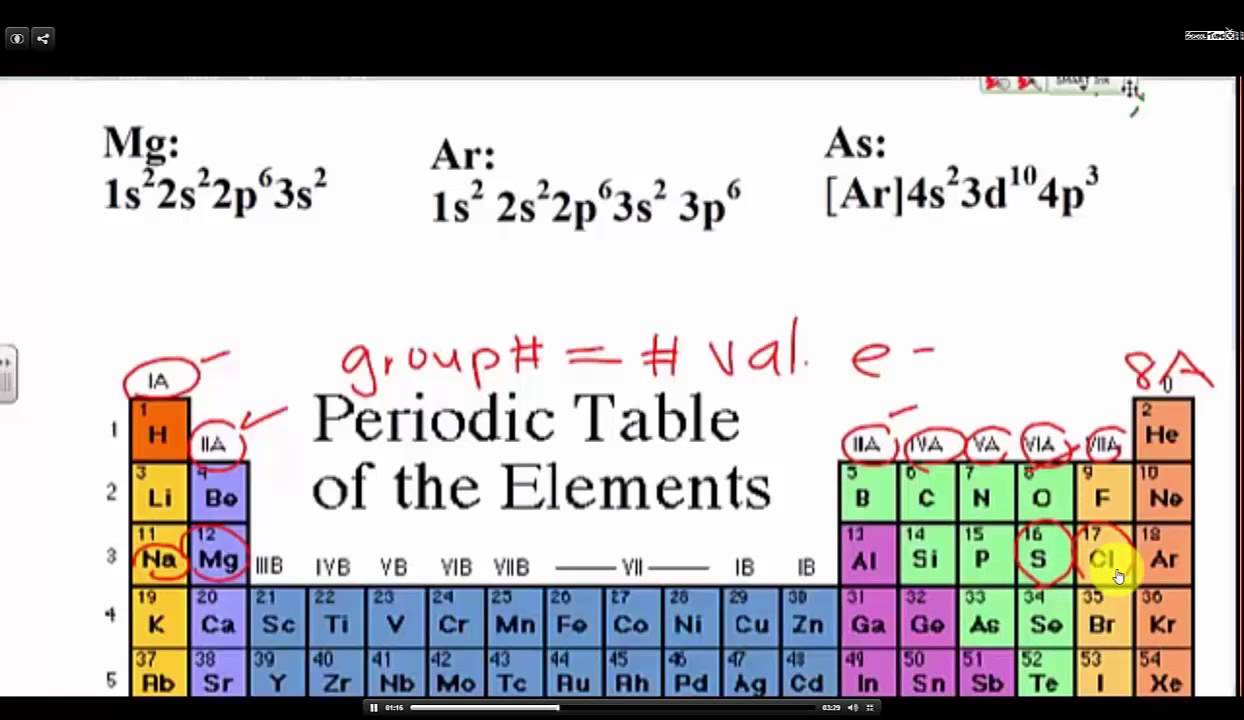
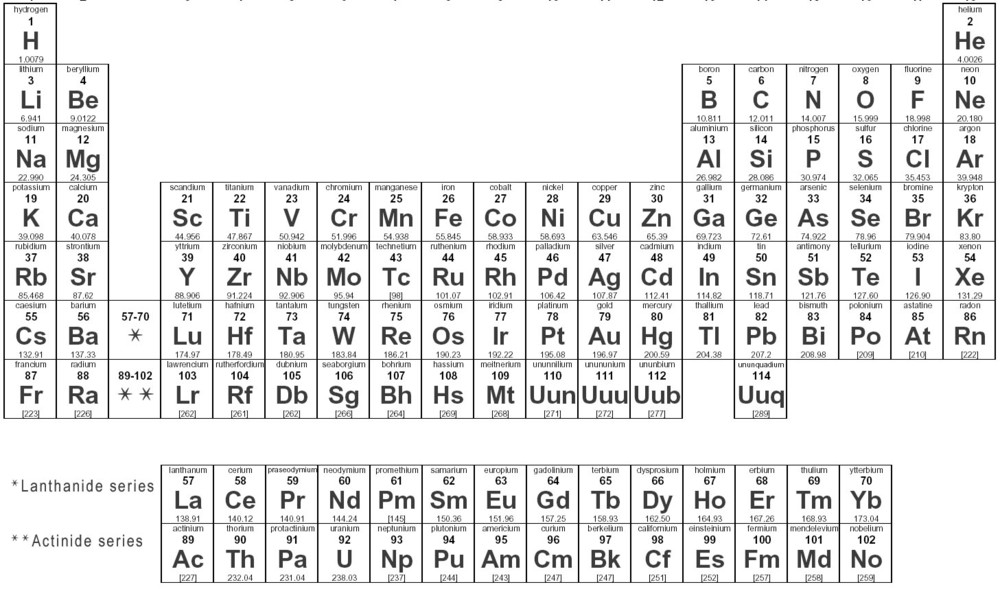
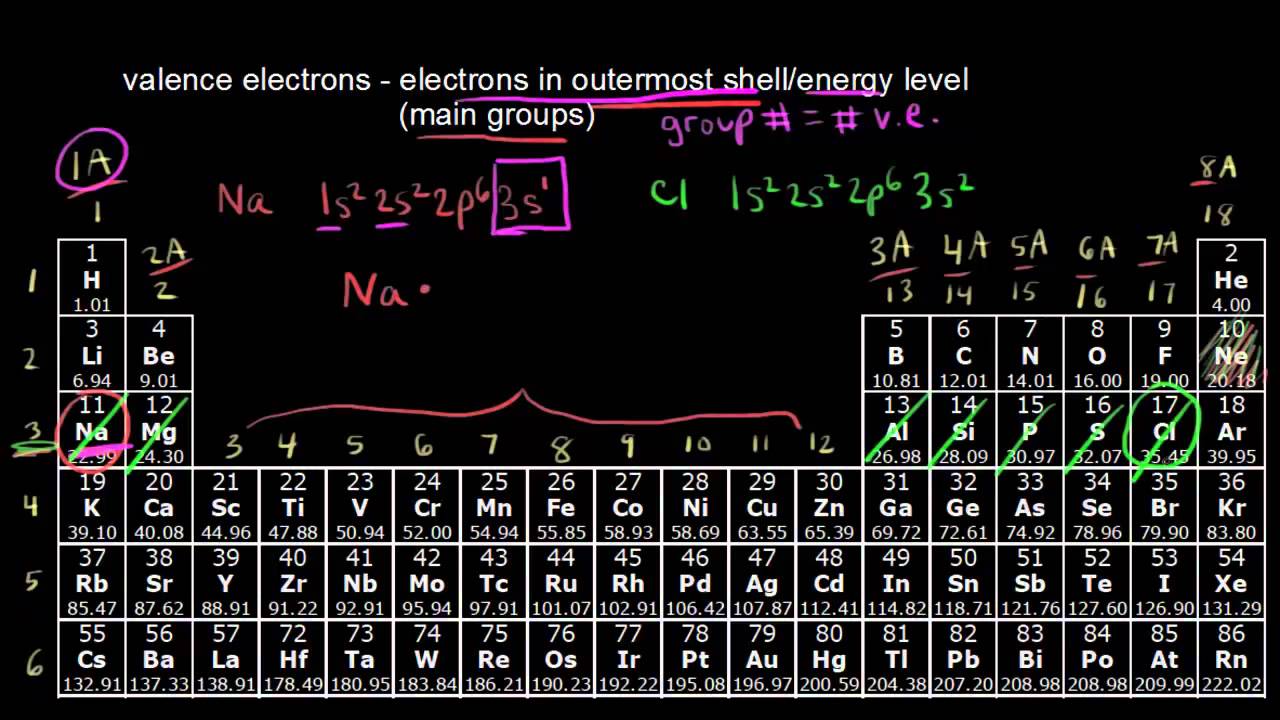
Label each column on the periodic table of elements from 1 to 18.
How to find out the valence electrons. More specifically, you have to see the group wise position of. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. More specifically, you have to see the.
To find out the valence electrons of phosphorus, you have to see the position of phosphorus in the periodic table. The electronegativity of oxygen is: From the periodic table to find out the valence electrons of tellurium,.
More specifically, you have to see the group wise position of. Valence electrons are located in the outermost shell of an atom. Χ = 3.44 in general, an atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the.
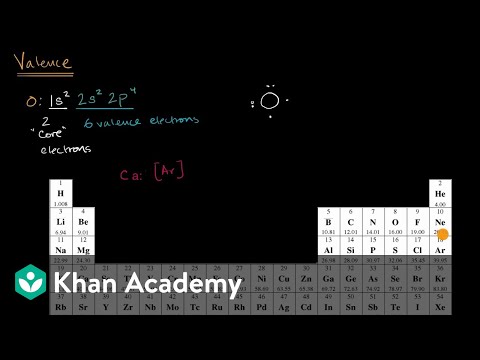
But for most of the. These are the valence electrons. How to find valence electrons from the electronic configuration?
The atomic number of nitrogen,. Generally, on a periodic table, all of the elements in a single vertical column will have the same number of. To find out the valence electrons of krypton, you have to see the position of krypton in the periodic table.
In order to find the valence electrons of tellurium atom (te), you can use two methods. How do you find valence electrons in class 9? For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell.



![How To Determine The Number Of Valence Electrons In An Element, Ion, Or Molecule [Quick And Easy] - Youtube](https://i.ytimg.com/vi/GEnqFx8MQ5w/maxresdefault.jpg)